- About us
- Research
- Students & Teaching
- Seminars & Events
- Directories
- Booking Rooms & Equipment
- עברית
Home » Prof. Etana Padan - The NhaA structural fold is critical for the antiporter functionality
All cells require concentration-regulation of intracellular H+ (pH) and Na+ to maintain homeostasis of these ions. Na+/H+ antiporters (membrane proteins exchanging Na+ for H+ across the cell membrane) are essential for this ions-homeostasis.
In 2005, we together with the group of H. Michel (Max-Planck, Frankfurt, Germany) determined the atomic crystal structure of NhaA, the main Escherichia coli Na+/H+ antiporter, opening the way to study the structure/function relationship of these membrane proteins.
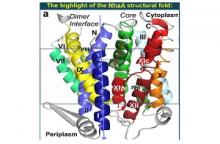
The structure revealed a unique structural fold dabbed the NhaA structural fold. Out of the 12 NhaA transmembrane segments (TMs), TMs III–V and X–XII are topologically inverted repeats with unwound TMs IV and XI forming the X shape characterizing the NhaA fold (Figure).
To test the functional importance of the NhaA fold we constructed a mutant with two Cysteine replacements across the crossing (D133C-T340C) which allowed intramolecular cross-linking under oxidizing conditions. We show that this cross-linking inhibits antiporter activity and impairs NhaA-dependent cell growth in high-salts and pH. The affinity purified D133C-T340C protein binds Li+ (the Na+ surrogate substrate of NhaA) under reducing conditions. The cross-linking traps the antiporter in an outward-facing conformation, blocking the antiporter activity cycle. Thus, the NhaA structural fold is critical for the antiporter functionality.
As many secondary transporters and other membrane proteins share the NhaA fold, including some involved in human diseases, our data have importance for both basic and clinical research.
This work was done in collaboration with Dr. Avraham Rimon and Dr. Hadar Amartely.
Read the paper - https://www.nature.com/articles/s41598-024-56425-3