- About us
- Research
- Students & Teaching
- Seminars & Events
- Directories
- Booking Rooms & Equipment
- עברית
Home » Prof. Julia Shifman reveals the nature of cold spots in protein-protein interactions
Protein–protein interactions (PPIs) play a key role in diverse biological functions including muscle contraction, signal transduction, cell metabolism, macromolecular assembly, and other cellular processes. Each PPI is characterized with a particular binding affinity (KD) that is compatible with PPI functional role in the cell. As such, PPIs that are responsible for cellular life/death decisions, such as for example, toxin/antitoxin interactions, bind to each other with extremely high affinities. PPIs that are involved in signaling usually possess medium binding affinities, allowing them to interact with their partners on the intermediate time scale. Transient and multi-specific interactions are characterized with weak binding affinities as quick dissociation of one protein from another is required for the correct functioning of such complexes.
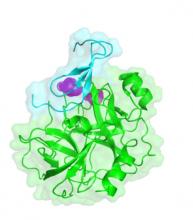
Proteins interact with each other through surface patches, termed binding interfaces, that are distinguishable from non-interface protein surface. PPI binding interfaces differ greatly in size, geometry, and physico-chemical properties. Molecular interactions across the binding interface, such as hydrophobic burial, hydrogen bonding, van der Waals, and electrostatic interactions largely determine PPI binding affinity. Within the binding interface, a few residues called hot spots contribute the majority of the binding free energy and are hence irreplaceable. In contrast, cold spots are occupied by suboptimal amino acids, providing possibility for affinity enhancement through mutations. We find that such cold spots could arise due to three scenarios: cavities at the binding interface, charged amino acids buried in the hydrophobic environment, or two amino acids of the same change that come in close proximity.
In a recent study, Sagara Gurusinghe (a Ph. D. student from the Shifman lab) and Ben Openheimer (an internship student in the lab) searched for cold spots in thousands of protein-protein complexes in the PDB. They observed that cold spots due to cavities are present in nearly all PPIs unrelated to their binding affinity. In contrast, charge-hydrophobic and same-charge interactions are observed with smaller frequency. They also found that most cold spots are located in the periphery of the binding interface, with high-affinity complexes showing fewer centrally located cold spots than low-affinity complexes. A larger number of cold spots is also found in non-cognate interactions compared to their cognate counterparts. Furthermore, the analysis reveals that cold spots are more frequent in homo-dimeric complexes compared to hetero-complexes, likely due to symmetry constraints imposed on sequences of homodimers. Finally, glycines, glutamates, and arginines are the most frequent amino acids appearing at cold spot positions. The study emphasizes the importance of cold spot positions to protein evolution and facilitates protein engineering studies directed at enhancing binding affinity and specificity in a wide range of applications.