- About us
- Research
- Students & Teaching
- Seminars & Events
- Directories
- Booking Rooms & Equipment
- עברית
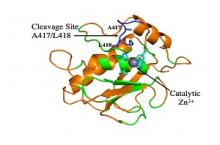
Home » Prof. Julia Shifman - Using protein engineering to develop new and more effective drugs against degenerative diseases, inflammatory diseases and cancer